NIA-Funded Active Alzheimer's and Related Dementias Clinical Trials and Studies | National Institute on Aging
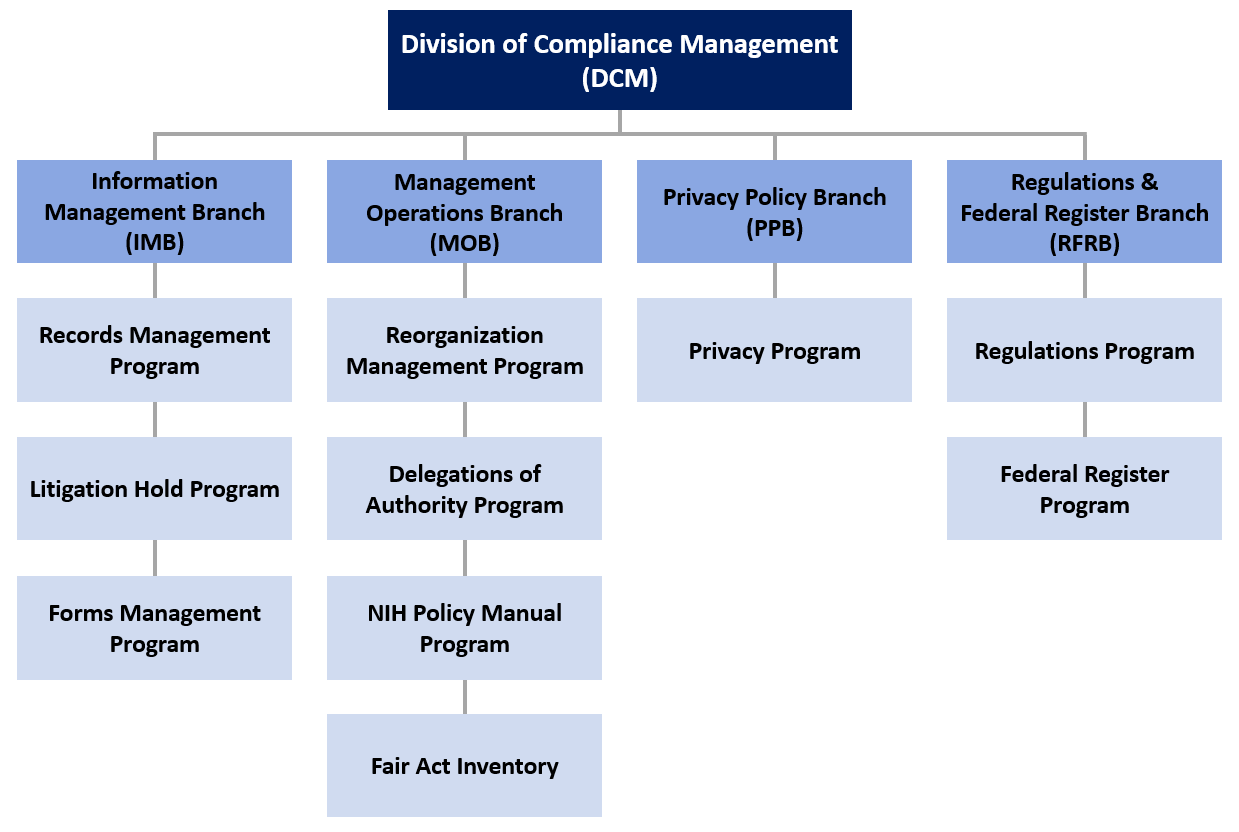
4 Questions For Researchers and Institutions Involved In Human Subjects Research – NIH Extramural Nexus

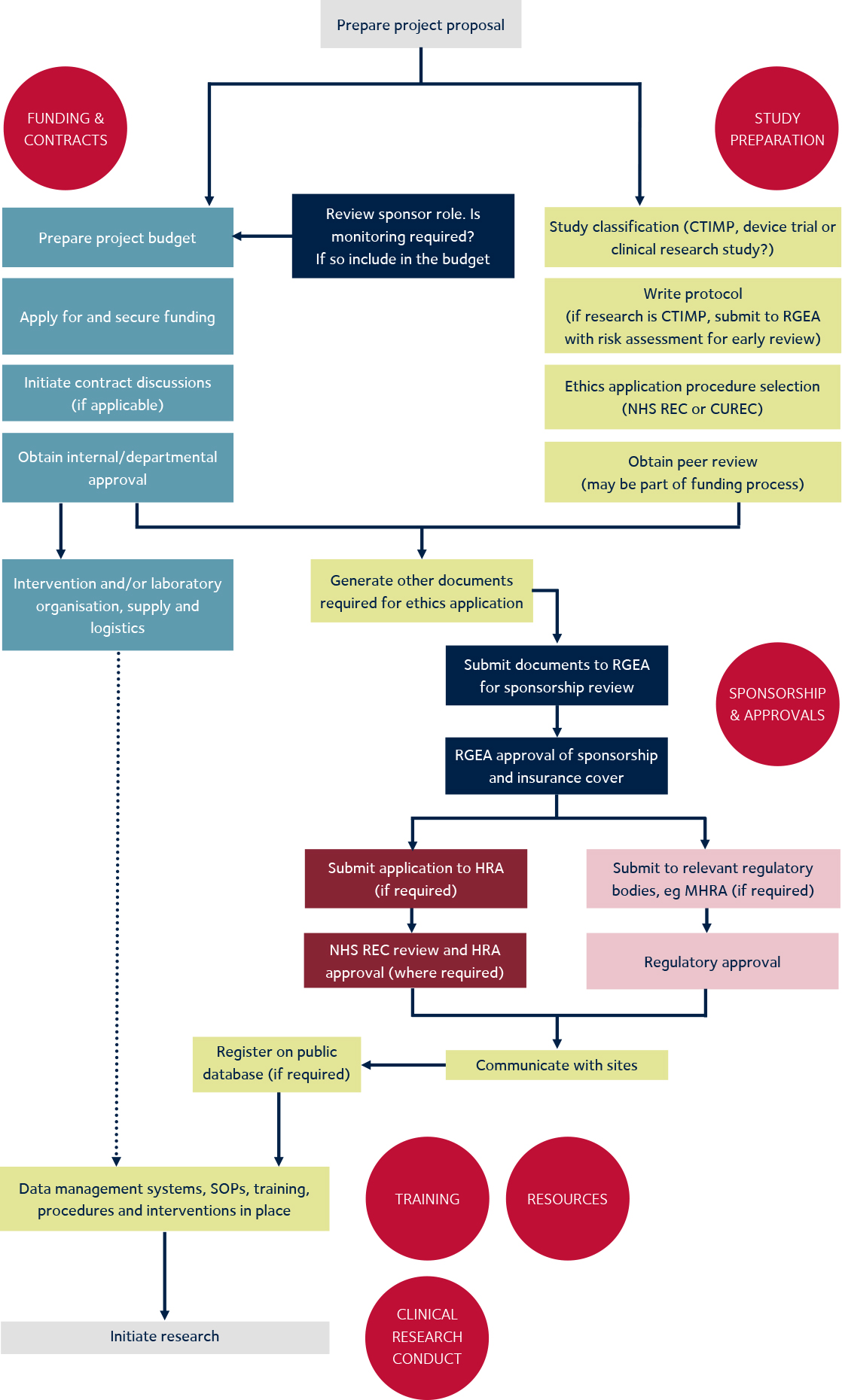
Guidance Document: Part C, Division 5 of the Food and Drug Regulations “Drugs for Clinical Trials Involving Human Subjects” (GUI-0100) - Canada.ca
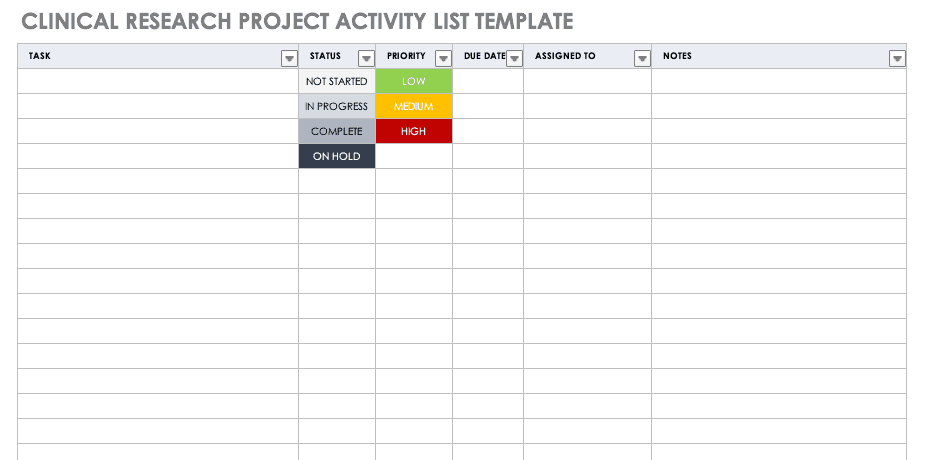
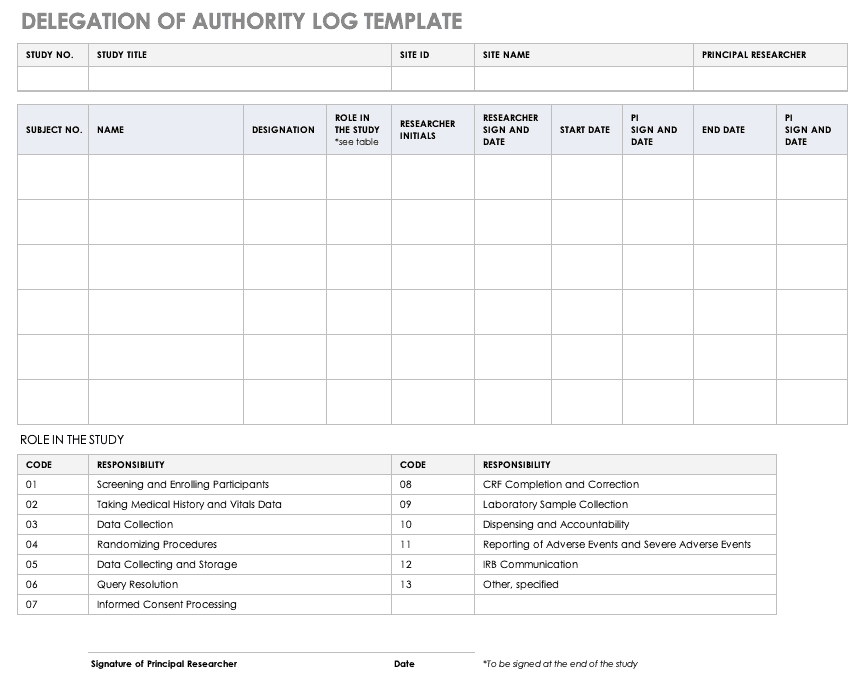
Transition from Inclusion Management System to New Human Subjects System (HSS) as of June 9, 2018 Purpose
Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan | PLOS Computational Biology